
Solutions for human and animal health
The OMERIN Group has been active in the medical industry since 2015 through its subsidiaries UNION PLASTIC and PRINCE MEDICAL. Our Business Unit, Medical Devices and Primary Pharmaceutical Packaging, currently represents 17 percent of the Group's turnover. We design and manufacture a wide range of innovative solutions in strictly controlled ISO9 / ISO8 / ISO7 environments for human health, animal health, the pharmaceutical industry and the diagnostics sector. Our production sites are EN ISO 13485 certified and handle a large number of EC certified programmes.
UNION PLASTIC
An internationally recognised specialist, UNION PLASTIC has been designing and manufacturing a complete range of innovative plastic solutions since 1955 for medical industries: dosing systems, primary pharmaceutical packaging, medical devices, animal health products, and consumables for in vitro diagnostics. The company is EN ISO 13485 and EN ISO 15378 certified. Product manufacturing is safeguarded by two back-up sites.
UNION PLASTIC has extensive know-how in plastics, with more than 100 high-speed presses between 100 and 300 T and a very large number of fully automated assembly, marking and packaging lines.
UNION PLASTIC is based in France, at Saint-Didier-en-Velay in the Haute-Loire Department. It has three production sites, including one in Tunisia with a total covered floor space of 15,000 m2.

PRINCE MEDICAL
With nearly 30 years of specialised experience in the Healthcare market, PRINCE MEDICAL designs and manufactures a full range of intrusive and non-intrusive single-use medical devices (class IIa, IIb, I) for Gastroenterology, Gynaecology, Urology and Medically-Assisted Procreation : injection needles, balloon catheters, nasobiliary tube, biopsy forceps, insemination catheters, oocyte puncture needles, and many more. Its EN ISO 13485 certification and total control over all industrial processes (injection moulding, extrusion, assembly of plastics and steel components, laser cutting, ultrasonic and laser welding, packaging, marking, sterilisation, etc.) guarantee top-quality products. PRINCE MEDICAL has more than 40 EC certified programmes.
PRINCE MEDICAL is based in Ercuis, France in the Oise Department. It has three production sites, including two in Tunisia, with a total covered floor space of 10,000 m2. The company has built its reputation on its technical know-how in the injection and extrusion of thermoplastics and polymers.

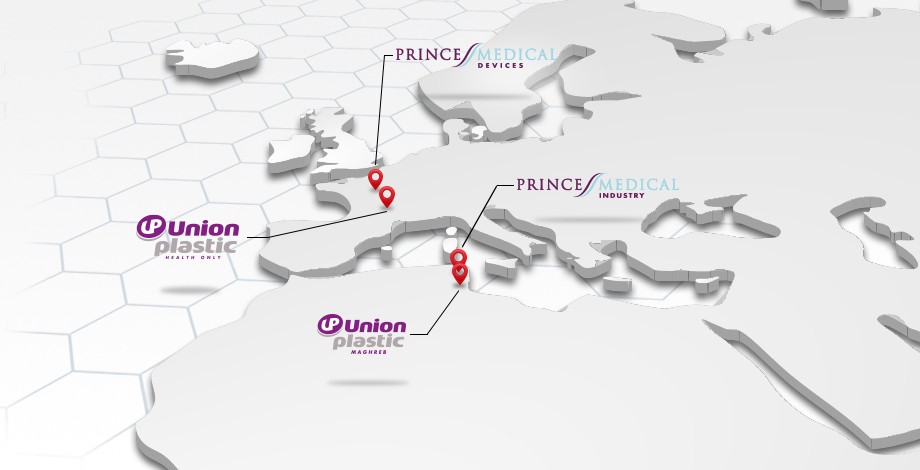
Our locations
The OMERIN Group production sites and sales offices
for Medical Devices and Primary Pharmaceutical Packaging.

 Nous utilisons des cookies pour mesurer la fréquentation de notre site et pour vous proposer une expérience utilisateur personnalisée. Par ce que le respect de votre vie privée est essentiel pour nous, nous vous laissons le choix de les accepter ou de les paramétrer.
Nous utilisons des cookies pour mesurer la fréquentation de notre site et pour vous proposer une expérience utilisateur personnalisée. Par ce que le respect de votre vie privée est essentiel pour nous, nous vous laissons le choix de les accepter ou de les paramétrer.